Copper Ii Sulfate With Sodium Hydroxide . word equation copper (ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water one. reaction of copper (ii) ion reacting with sodium hydroxide. Cu + 2 (aq) + 2oh − (aq) − ⇀ ↽ − cu(oh) 2(s) the precipitate. copper (ii) sulfate and sodium hydroxide reaction | cuso4 + naoh |. Asked 7 years, 9 months ago. i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate,. two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. When copper sulfate cuso 4 and sodium hydroxide naoh reacts, a blue colored precipitate of copper hydroxide cu. sodium hydroxide precipitates copper (ii) hydroxide: in this video we'll balance the equation cuso4 + naoh = cu (oh)2 + na2so4.
from www.youtube.com
word equation copper (ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water one. copper (ii) sulfate and sodium hydroxide reaction | cuso4 + naoh |. reaction of copper (ii) ion reacting with sodium hydroxide. Cu + 2 (aq) + 2oh − (aq) − ⇀ ↽ − cu(oh) 2(s) the precipitate. Asked 7 years, 9 months ago. two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate,. sodium hydroxide precipitates copper (ii) hydroxide: When copper sulfate cuso 4 and sodium hydroxide naoh reacts, a blue colored precipitate of copper hydroxide cu. in this video we'll balance the equation cuso4 + naoh = cu (oh)2 + na2so4.
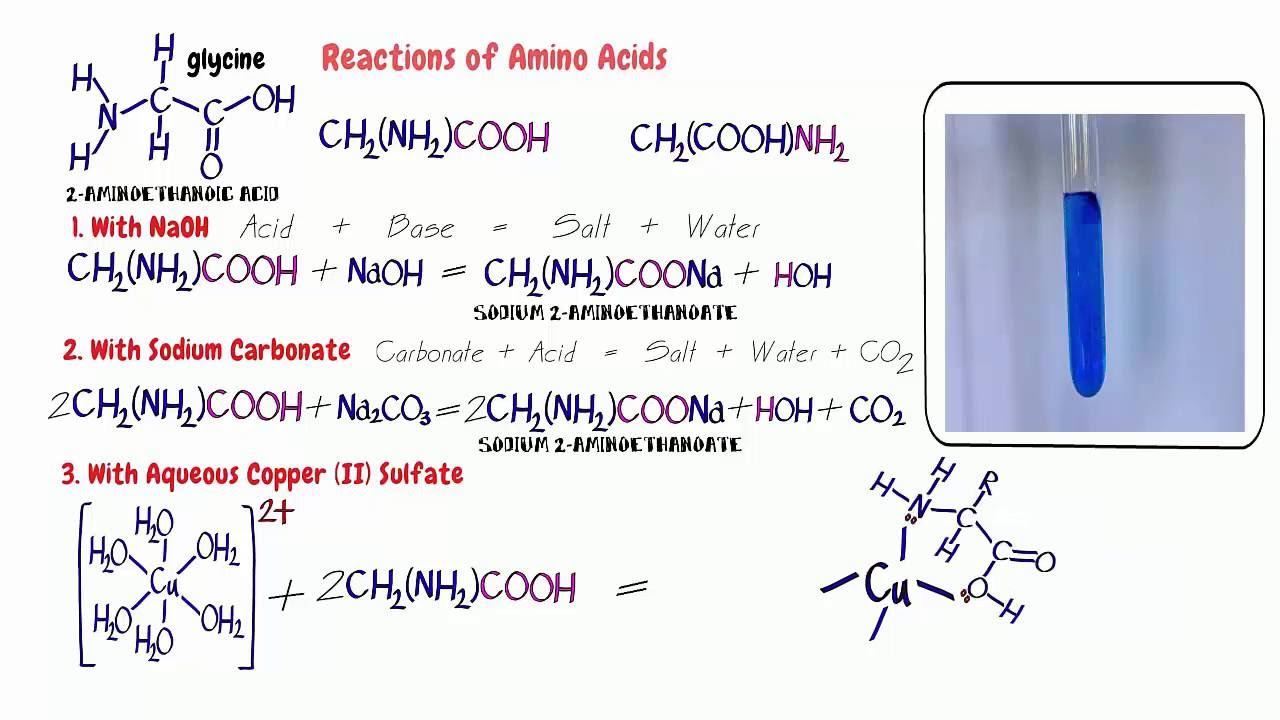
Amino Acids 3. Reactions with sodium hydroxide, sodium carbonate
Copper Ii Sulfate With Sodium Hydroxide i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate,. sodium hydroxide precipitates copper (ii) hydroxide: Asked 7 years, 9 months ago. two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. word equation copper (ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water one. i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate,. reaction of copper (ii) ion reacting with sodium hydroxide. copper (ii) sulfate and sodium hydroxide reaction | cuso4 + naoh |. Cu + 2 (aq) + 2oh − (aq) − ⇀ ↽ − cu(oh) 2(s) the precipitate. When copper sulfate cuso 4 and sodium hydroxide naoh reacts, a blue colored precipitate of copper hydroxide cu. in this video we'll balance the equation cuso4 + naoh = cu (oh)2 + na2so4.
From www.chegg.com
Solved REACTION 5 COPPER SULFATE (CuSO) AND SODIUM HYDROXIDE Copper Ii Sulfate With Sodium Hydroxide Asked 7 years, 9 months ago. Cu + 2 (aq) + 2oh − (aq) − ⇀ ↽ − cu(oh) 2(s) the precipitate. word equation copper (ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water one. copper (ii) sulfate and sodium hydroxide reaction | cuso4 + naoh |. two moles of aqueous sodium. Copper Ii Sulfate With Sodium Hydroxide.
From www.youtube.com
The Reaction Between Copper (II) Nitrate and Sodium Hydroxide YouTube Copper Ii Sulfate With Sodium Hydroxide Asked 7 years, 9 months ago. two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. copper (ii) sulfate and sodium hydroxide reaction | cuso4 + naoh |. word equation copper (ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water one. When copper. Copper Ii Sulfate With Sodium Hydroxide.
From www.doubtnut.com
Copper [II] sulphate solution. Reacts with sodium hydroxide solution to Copper Ii Sulfate With Sodium Hydroxide i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate,. Asked 7 years, 9 months ago. sodium hydroxide precipitates copper (ii) hydroxide: in this video we'll balance the equation cuso4 + naoh = cu (oh)2 + na2so4. Cu + 2 (aq) + 2oh − (aq) − ⇀ ↽ − cu(oh) 2(s) the. Copper Ii Sulfate With Sodium Hydroxide.
From www.alamy.com
Zinc Powder, Copper(II) sulfate and Sodium Hydroxide Pellets in test Copper Ii Sulfate With Sodium Hydroxide i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate,. When copper sulfate cuso 4 and sodium hydroxide naoh reacts, a blue colored precipitate of copper hydroxide cu. reaction of copper (ii) ion reacting with sodium hydroxide. Asked 7 years, 9 months ago. word equation copper (ii) sulfate pentahydrate + sodium hydroxide. Copper Ii Sulfate With Sodium Hydroxide.
From brainly.in
What are the products of Copper sulphate + sodium hydroxide? Brainly.in Copper Ii Sulfate With Sodium Hydroxide word equation copper (ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water one. reaction of copper (ii) ion reacting with sodium hydroxide. Asked 7 years, 9 months ago. When copper sulfate cuso 4 and sodium hydroxide naoh reacts, a blue colored precipitate of copper hydroxide cu. two moles of aqueous sodium hydroxide. Copper Ii Sulfate With Sodium Hydroxide.
From www.alamy.com
Chemical ingredient in hexagonal molecular shaped container. Sodium Copper Ii Sulfate With Sodium Hydroxide copper (ii) sulfate and sodium hydroxide reaction | cuso4 + naoh |. When copper sulfate cuso 4 and sodium hydroxide naoh reacts, a blue colored precipitate of copper hydroxide cu. reaction of copper (ii) ion reacting with sodium hydroxide. two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of. Copper Ii Sulfate With Sodium Hydroxide.
From www.numerade.com
Copper(II) sulfate and sodium hydroxide solutions… Copper Ii Sulfate With Sodium Hydroxide reaction of copper (ii) ion reacting with sodium hydroxide. word equation copper (ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water one. Cu + 2 (aq) + 2oh − (aq) − ⇀ ↽ − cu(oh) 2(s) the precipitate. sodium hydroxide precipitates copper (ii) hydroxide: When copper sulfate cuso 4 and sodium hydroxide. Copper Ii Sulfate With Sodium Hydroxide.
From www.chegg.com
Solved copper (II) sulfate + sodium hydroxide → Blue Copper Ii Sulfate With Sodium Hydroxide Asked 7 years, 9 months ago. copper (ii) sulfate and sodium hydroxide reaction | cuso4 + naoh |. sodium hydroxide precipitates copper (ii) hydroxide: in this video we'll balance the equation cuso4 + naoh = cu (oh)2 + na2so4. i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate,. two. Copper Ii Sulfate With Sodium Hydroxide.
From www.chegg.com
Solved Copper(II) sulfate + Sodium hydroxide Blue gelatinous Copper Ii Sulfate With Sodium Hydroxide two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. word equation copper (ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water one. copper (ii) sulfate and sodium hydroxide reaction | cuso4 + naoh |. When copper sulfate cuso 4 and sodium hydroxide. Copper Ii Sulfate With Sodium Hydroxide.
From www.slideserve.com
PPT Evidence of Chemical Change Laboratory PowerPoint Presentation Copper Ii Sulfate With Sodium Hydroxide Asked 7 years, 9 months ago. two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. word equation copper (ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water one. sodium hydroxide precipitates copper (ii) hydroxide: reaction of copper (ii) ion reacting with. Copper Ii Sulfate With Sodium Hydroxide.
From www.slideshare.net
Acids And Bases Copper Ii Sulfate With Sodium Hydroxide Cu + 2 (aq) + 2oh − (aq) − ⇀ ↽ − cu(oh) 2(s) the precipitate. two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. copper (ii) sulfate and sodium hydroxide reaction | cuso4 + naoh |. When copper sulfate cuso 4 and sodium hydroxide naoh reacts, a. Copper Ii Sulfate With Sodium Hydroxide.
From exorggbgv.blob.core.windows.net
Copper Ii Sulfate And Ammonium Hydroxide Net Ionic Equation at Jennifer Copper Ii Sulfate With Sodium Hydroxide Cu + 2 (aq) + 2oh − (aq) − ⇀ ↽ − cu(oh) 2(s) the precipitate. in this video we'll balance the equation cuso4 + naoh = cu (oh)2 + na2so4. two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. copper (ii) sulfate and sodium hydroxide reaction. Copper Ii Sulfate With Sodium Hydroxide.
From exovhomju.blob.core.windows.net
Copper (Ii) Chloride And Sodium Hydroxide Reaction at Marie Croom blog Copper Ii Sulfate With Sodium Hydroxide word equation copper (ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water one. sodium hydroxide precipitates copper (ii) hydroxide: Cu + 2 (aq) + 2oh − (aq) − ⇀ ↽ − cu(oh) 2(s) the precipitate. copper (ii) sulfate and sodium hydroxide reaction | cuso4 + naoh |. When copper sulfate cuso 4. Copper Ii Sulfate With Sodium Hydroxide.
From www.dreamstime.com
Chemical Ingredient in Hexagonal Molecular Shaped Container. Sodium Copper Ii Sulfate With Sodium Hydroxide When copper sulfate cuso 4 and sodium hydroxide naoh reacts, a blue colored precipitate of copper hydroxide cu. in this video we'll balance the equation cuso4 + naoh = cu (oh)2 + na2so4. two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. sodium hydroxide precipitates copper (ii). Copper Ii Sulfate With Sodium Hydroxide.
From www.youtube.com
Copper(II) Sulfate & Sodium Phosphate YouTube Copper Ii Sulfate With Sodium Hydroxide reaction of copper (ii) ion reacting with sodium hydroxide. i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate,. word equation copper (ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water one. sodium hydroxide precipitates copper (ii) hydroxide: Asked 7 years, 9 months ago. Cu +. Copper Ii Sulfate With Sodium Hydroxide.
From www.youtube.com
Amino Acids 3. Reactions with sodium hydroxide, sodium carbonate Copper Ii Sulfate With Sodium Hydroxide sodium hydroxide precipitates copper (ii) hydroxide: two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. Cu + 2 (aq) + 2oh − (aq) − ⇀ ↽ − cu(oh) 2(s) the precipitate. word equation copper (ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate +. Copper Ii Sulfate With Sodium Hydroxide.
From www.alamy.com
Adding NH4OH Ammonium Hydroxide to CuSO4 Copper II sulfate to Yield Copper Ii Sulfate With Sodium Hydroxide Cu + 2 (aq) + 2oh − (aq) − ⇀ ↽ − cu(oh) 2(s) the precipitate. i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate,. sodium hydroxide precipitates copper (ii) hydroxide: When copper sulfate cuso 4 and sodium hydroxide naoh reacts, a blue colored precipitate of copper hydroxide cu. Asked 7 years,. Copper Ii Sulfate With Sodium Hydroxide.
From www.dreamstime.com
Sodium Hydroxide Pellets, Zinc Granules and Copper(II) Sulfate in Test Copper Ii Sulfate With Sodium Hydroxide reaction of copper (ii) ion reacting with sodium hydroxide. Asked 7 years, 9 months ago. i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate,. word equation copper (ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water one. Cu + 2 (aq) + 2oh − (aq) −. Copper Ii Sulfate With Sodium Hydroxide.